FAYETTEVILLE, Ark. - Nov. 2, 2021 - PRLog -- Lineus Medical announces the latest addition to its executive team with the hiring of Ryan Brick of Castle Rock, Colorado. Ryan joins Lineus Medical as Vice President of Sales and leads all sales related efforts for the company.
Ryan has over 15 years of experience working in vascular access medical device sales. Before becoming Vice President of Sales at Lineus Medical, he led sales for the West Region at RyMed Technologies. While there, he spearheaded sales in large healthcare systems introducing new vascular access product products. Prior to RyMed Technologies, he was a part of the initial sales team that launched Curos Disinfecting End Caps at Ivera Medical, which resulted in Ivera Medical being sold to 3M in 2015. Ryan also spent 6 years working for ICU Medical as a Product Specialist, where he helped bring four new products to the vascular access market. Ryan has a degree in Business Management and was a member of the Professional Golfers' Association of America.
"We are very excited to have Ryan join our team," said Vance Clement, CEO of Lineus Medical. "His experience in the vascular access arena will greatly energize our sales efforts during this crucial phase of the company. After securing FDA clearance for SafeBreak Vascular in the summer of 2021, the company's focus has shifted to expanding distribution and driving sales. Ryan is essential to our effort to continue building sales momentum."
Ryan Brick commented, "After having worked for 2 previous startups and launching several new vascular access products, I believe that I've joined the right organization for my future success and an organization that has a terrific first product, SafeBreak Vascular. I am looking forward to leading the sales effort for making this new and innovative device the standard of care for peripheral IVs."
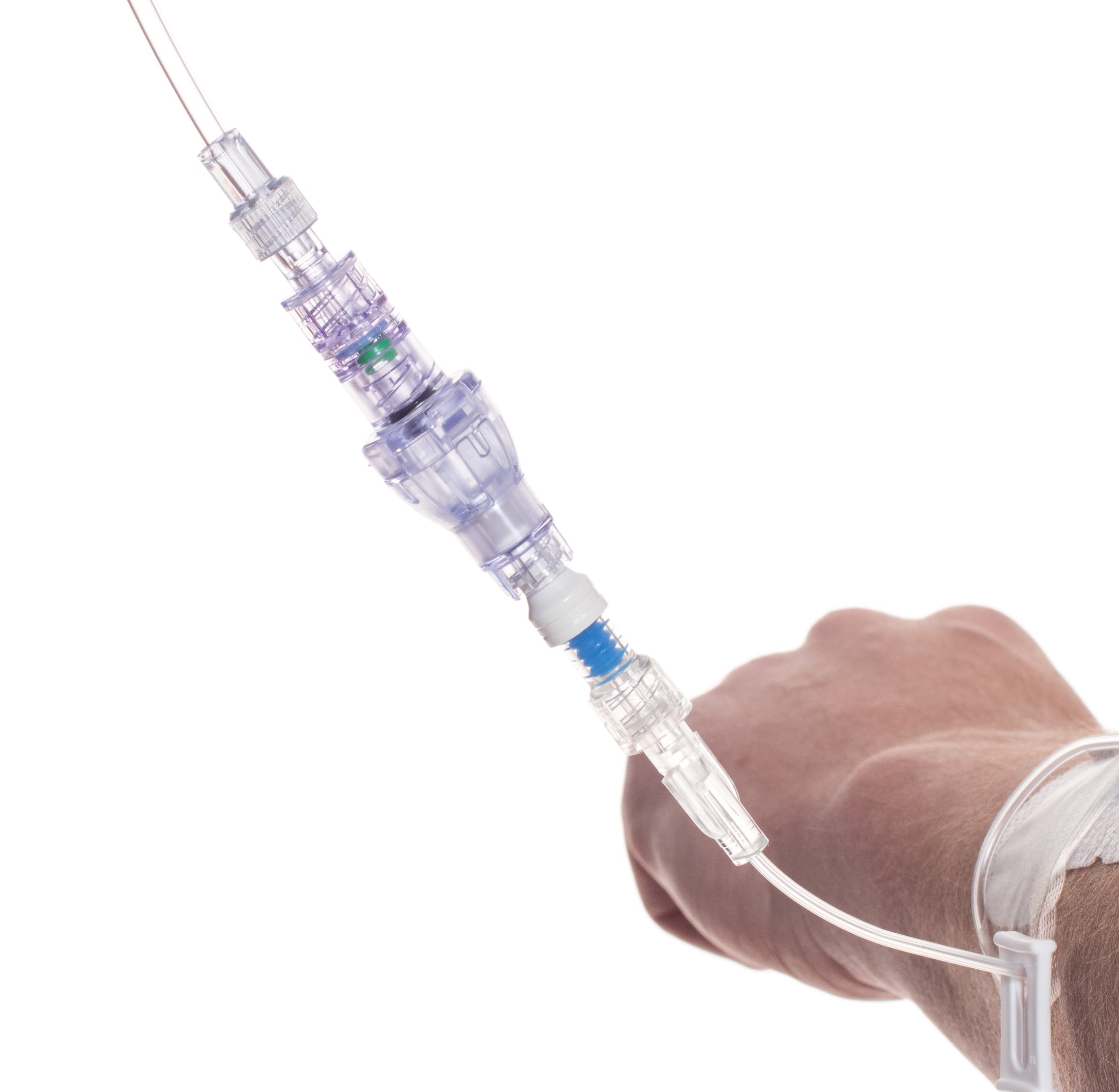
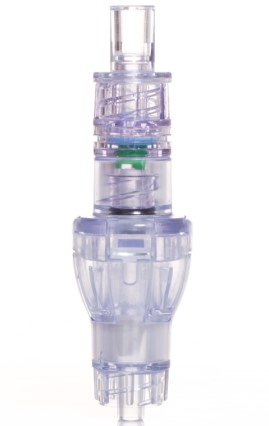
SafeBreak Vascular is a break-away connector that fits into any peripheral IV line on any standard luer lock in the world, although currently it is only cleared for use in the United States. The device separates when a damaging force is placed on an IV line, therefore reducing irritation of the vein and the chances of the IV dislodging. When the device separates, valves on each side of the device close to stop the flow of medication from the pump and blood flow from the patient's IV catheter. The separated SafeBreak can be replaced with a new, sterile SafeBreak and the IV restarted without the need for another needlestick for the patient. In a January 2021 randomized controlled trial, SafeBreak Vascular was shown to reduce IV complications requiring an IV restart by 44%.1
SafeBreak Vascular reduces the failure of peripheral IV lines and the negative cascade of events that occurs when an IV restart is required. Over 235M peripheral IVs are successfully placed in the United States each year and over 2B globally.2 Peripheral IVs fail 46% of the time before their intended use is complete according to the clinical literature and cost the US healthcare system over $5B annually.
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a unique, patented, break-away technology that is designed to work with all types of medical tubing. Complications with feeding tubes, IV lines, urinary catheters and any other type of medical tubing may be reduced when a force-activated separation device separates to protect the patient. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn or Twitter.
MKG-0120 Rev 00
1D.I.P.P.E.R Study - Data on file.
2Helm, R.E., et al., Accepted but Unacceptable: Peripheral IV Catheter Failure. Journal of Infusion Nursing. 2015;38(3): 189-203
Contact
Kelly Patterson
***@lineusmed.com
Photos: (Click photo to enlarge)![]()

Read Full Story - Lineus Medical adds VP of Sales, Ryan Brick | More news from this source
Press release distribution by PRLog
Lineus Medical adds VP of Sales, Ryan Brick
November 02, 2021 at 07:00 AM EDT

